Thursday, December 3, 2009
Lesson One
Lesson Plan One
Teacher: Angela Schneider Course: Physics Grade: 11-12
Topic: Nuclear Energy
CLE(s): Describe how changes in the nucleus of an atom during a nuclear reaction (i.e., nuclear decay, fusion, fission) result in emission of radiation).
Identify the role of nuclear energy as it serves as a source of energy for the Earth, stars, and human activity (e.g., source of electromagnetic radiation, thermal energy within mantle, nuclear power plants, fuel for stars).
Objectives: The student will identify different types of radiation.
The student will describe the half-life process and demonstrate how they are calculated.
Materials needed: fill-in notes for each student, writing utensils
Introduction:
Ask students what they think of when they hear the word ‘radiation’. Write key points on the board.
Radiation is all around us, yet we can’t see it, feel it, smell it, or taste it. It comes from the earth itself, space, and man-made sources.
Content:
Present content to the class, writing key points on the board. Students should follow along and complete the provided notes.
Radiation is produced by unstable atoms. Unstable atoms eventually decay into stable atoms and the energy produced as the atom decays is radiation.
Sample decay chain:
Uranium-238 > Thorium-230 > Radium-226 > Radon-218 > Bismuth 214 > Lead-206
No one knows exactly how a radioactive element will decay, but there is a known pattern describing how long it will take a particular atom to lose half its radioactivity called the half-life. Formula used to calculate half-life:
A_E=A_o×〖0.5〗^(t/t_□(1/2) )
A_E: the amount of the substance remaining
A_o: the original amount of the substance
t: elapsed time
t_(1/2): half-life of the particular substance
Example: If you originally had 157 grams of carbon-14 and the half-life of carbon-14 is 5730 years, how much would there be after 2000 years?
Three different types of radiation:
Alpha radiation: alpha particles: emitted from unstable isotopes +2 charge, slow, easily stopped (paper, skin), internal hazard (damages tissues and cells)
Beta radiation: beta particles: high energy electrons, faster and lighter than alpha particles, can travel through skin, internal and external hazard, blocked by thin layers of metals and plastic
Gamma radiation: gamma rays: high energy light – part of the electromagnetic spectrum, travels at the speed of light, internal and external hazard, blocked by thick materials like cement or steel
Alpha, beta, and gamma radiation are classified as ionizing radiation, which is especially dangerous because it can change the chemical makeup of many living things. All exposure to radiation will have biological effects, but exposure to large amounts of radiation can cause genetic defects or cancer, causes ionization of atoms, which affect molecules, which may affect cells, which may affect tissues, which may affect the whole body.
Conclusion:
Tomorrow we will do two labs, focusing on radiation.
Lesson One: Notes
RADIATION NOTES
I. ____________________ is produced by unstable atoms.
A. Unstable atoms are ____________________________________________________
______________________________________________________________________
______________________________________________________________________
______________________________________________________________________
B. Unstable atoms undergo a ___________________ process. The energy that is produced is called radiation.
1. Decay chain:
Uranium-238 > ____________________________ > ______________________________ >
Radon-218 > ¬¬¬¬¬¬¬¬¬¬¬¬¬¬¬¬¬¬____________________________ > ______________________________
2. No one knows exactly how a particular atom will decay, but there is a known pattern describing _________________________________________________
________________________________________________________________
called the ________________________________________________________
a. Formula used to calculate the amount of decay:
A_E=A_o×〖0.5〗^(t/t_□(1/2) )
1. A_E: __________________________________________
2. A_o: __________________________________________
3. t:____________________________________________
4. t_(1/2): __________________________________________
b. If you originally had 157 grams of carbon-14 and the half-life of carbon-14 is 5730 years, how much would there be after 2000 years?
II. Types of radiation
A. Alpha radiation
1. Comprised of _____________________________, which have a charge of +2.
2. Slow, easily stopped by ___________________________________________,
_________________________ hazard – can damage ______________________
_________________________________________________________________
B. Beta radiation
1. Comprised of _______________________________, which are ___________
_________________________________________________________________
and are ________________________________________ than alpha particles.
2. Can travel through _________________, making it an ___________________
and __________________________ hazard.
3. Can be blocked by _______________________________________________
C. Gamma radiation
1. Comprised of ______________________________, which are _____________
_____________________________________________________ .
a. Part of the ________________________________________________.
b. Travel at _________________________________________________,
which is _____________________________.
2. Can be blocked by ________________________________________________
making it an _________________________ and __________________________
hazard.
D. Alpha, beta, and gamma radiation are all forms of _____________________________
________________, which is dangerous because ________________________________
________________________________________________________________________
III. Exposure to radiation will have _________________________________________________
A. Large amounts of radiation can cause _______________________________________
________________________________________________________________________
1. Which are caused by the _________________ of atoms.
2. Creates a chain of events that can eventually affect the ___________________
Atoms > ________________ > ________________ > ________________ > Whole body
Lesson Two
Lesson Plan Two
*Experiments from the U.S. Nuclear Regulatory Commission
Teacher: Angela Schneider Course: Physics Grade: 11-12
Topic: Nuclear Energy
CLE(s): Make qualitative and quantitative observations using the appropriate senses, tools and equipment to gather data (e.g., microscopes, thermometers, analog and digital meters, computers, spring scales, balances, metric rulers, graduated cylinders).
Identify the role of nuclear energy as it serves as a source of energy for the Earth, stars, and human activity (e.g., source of electromagnetic radiation, thermal energy within mantle, nuclear power plants, fuel for stars).
Objectives: The student will investigate the ‘footprints’ of radiation, using The Cloud Chamber experiment.
The student will detect and measure radiation using a Geiger counter.
Materials needed: ‘The Cloud Chamber’ and ‘Using a Geiger Counter’ experiment sheets (per student); small transparent container with transparent lid, flat black spray paint, blotter paper, pure ethyl alcohol, radioactive source, masking tape, dry ice, styrofoam square, flashlight, gloves or tongs to handle the dry ice (per lab group); Geiger counter, radioactive sources, shielding materials (per lab group)
Phase 1: Introduction: Orient students to the problem.
Pass out and go over experiment sheets.
Phase 2: Organize students for study.
Students will work in their pre-determined lab groups.
Phase 3: Assist group investigations
Walk around the classroom as students perform the experiments, helping when necessary.
Phase 4: Analyze and evaluate the problem-solving process.
Students will answer post-lab questions.
Lesson Two: The Cloud Chamber
The Cloud Chamber
While radiation cannot be seen, the cloud chamber allows you to see the tracks it leaves in a dense gas.
Materials
small transparent container with transparent lid
flat black spray paint
blotter paper
pure ethyl alcohol
radioactive source
masking tape
dry ice
styrofoam square
flashlight
gloves or tongs to handle the dry ice
First, paint the bottom of the container with black paint and let it dry. Then cut the blotter paper into a strip about as wide as the height of the container. Cut two windows in the strip, as shown, and place it against the inside of the container.
Directions:
Pour enough ethyl alcohol into the cloud chamber to cover the bottom of the container. The blotter paper will absorb most of it.
Place the radioactive source in the cloud chamber and seal the lid with tape.
Place the cloud chamber on the dry ice to super-chill it. Wait about five minutes. Darken the room. Shine the flashlight through the windows of the chamber while looking through the lid. You should see "puffs" and "trails" coming from the source. These are the "footprints" of radiation as it travels through the alcohol vapor. The vapor condenses as the radiation passes through. This is much like the vapor trail left by high flying jets.
Do you see radiation in the cloud chamber? ________
Other Ideas To Explore
Try to identify these footprints:
•Alpha: sharp tracks about 1 cm long
•Beta: thin tracks 3 cm to 10 cm long
•Gamma: faint, twisting and spiraling tracks
Caution: Dry ice should be handled very carefully! It can burn unprotected skin.
Lesson Two: Using a Geiger Counter
Using a Geiger Counter
How radioactive are different materials?
Materials
Geiger counter
Radioactive sources such as:
cloisonne jewelry
commercially available source from a science supply house
luminescent clock face
Shielding materials such as:
paper
aluminum foil
brick
jar of water
piece of wood
glass pane
sheet of lead
Directions:
1.One at a time, test each item that is a source of radioactivity by placing the source 2 inches from the Geiger counter probe. Which item has the highest reading?___________
The lowest?__________________________
2.Place the radioactive source that had the highest reading 2 inches from the
Geiger counter probe. One at a time, test each of your shielding materials by
placing them between the source and the counter. Do you think the density of the
shield is important?______________________________
Why?_________________________________________
Lesson Two: Post Lab Questions
Post lab questions
1.What materials are best for shielding?
2.Gamma radiation, a powerful type of radiation emitted from some radioactive isotopes, has no weight. What other types of radiation particles have no weight?
3.Because you could not see the radiation, what kind of observation did you experience?
4.What is happening to the radioactive source?
5.What radiation "footprints" did you see? Describe them.
6.Why do we measure radiation exposure?
7.When you use a Geiger counter to survey a radioactive substance, why is it important to know what the background radiation level is?
8.Has anyone you know been helped or harmed by radiation?
Lesson Three
Lesson Plan Three
http://www.paec.org/progressenergygrant/lesson_plans.html
Title: Nuclear Energy Transformed
Grades: 9-12
Subject Area(s): Science and Reading
Purpose of the Lesson:
To increase students’ knowledge of nuclear fission and fusion; how they are alike, how they differ and how they may be used in generating electricity
Essential Question(s):
What are nuclear fission and fusion?
How are they alike and how are they different?
How is the fission process used in a nuclear reactor?
Objectives:
Upon the completion of the discussion, activities, and reading assignments, students will:
1. Define nuclear energy, nuclear fission, nuclear fusion and nuclear chain reaction.
2. Describe the process of nuclear fission and nuclear fusion and evaluate how the
processes are alike and how they are different.
3 Explain how nuclear reactors are used to produce electrical energy.
4. Develop a timeline for the advancement of nuclear energy.
Materials:
Graphic Organizers: KWL, Venn diagram
These may be accessed at: http://www.eduplace.com/graphicorganizer/
Computer and internet access for word processing, PowerPoint application, and accessing
resource materials
Visuals: Fusion reaction, fission reaction, nuclear reactor
Writing Journals/notebooks, Reflection sheets
Articles: “Nuclear Fission” and “Nuclear Fusion” which may be accessed at:
http://www.howstuffworks.com/nuclear-power1.htm
http://science.howstuffworks.com/fusion-reactor1.htm
Websites:
Nuclear Power Plant Demonstration
http://www.ida.liu.se/~her/npp/demo.html
How Nuclear Power Works
http://www.howstuffworks.com/nuclear-power2.htm
Nuclear Fission
http://www.howstuffworks.com/nuclear-power1.htm
Nuclear Fusion Animation
http://web.jjay.cuny.edu/~acarpi/NSC-2/NuclearFusion.html
Lesson Procedures:
1. Introduce the lesson - Using a KWL chart, ask for student responses for “What I
Know” about Nuclear Fission and Fusion.
2. Group students into cooperative groups to share their responses to “What I Know.”
Complete another KWL to capture each group’s knowledge. Each group will share
any responses, not already listed on the board.
NOTE: Any student misconceptions may be noted as “What I Need to Know.”
3. Give students a copy of the “Fission - Fusion Anticipation Guide” to complete, based
upon the groups’ responses on the KWL. The guide is composed of selected
questions from the articles, to be completed as a before-reading activity.
Students will record responses on the left-hand side of the anticipation guide.
4. Provide articles and instruct students to complete the right side of the anticipation
guide as they read the article. This is a during-reading activity.
5. After students have read the article, ask students to read selected questions from the
anticipation guide, determine whether the statement is true or false, and discuss the
rationale for their response.
6. REVIEW Nuclear Fission using the information available at the following Web site:
http://www.howstuffworks.com/nuclear-power1.htm
7. REVIEW Nuclear Fusion using the information available at the following Web site:
http://web.jjay.cuny.edu/~acarpi/NSC-2/NuclearFusion.html
8. As a formative assessment, ask students to complete a Venn diagram, comparing and
contrasting nuclear fission and nuclear fusion.
9. Allow students to use computers to interact with an animated nuclear energy reactor
to gain understanding of reactor structure and the working process. If students do not
have classroom access to computers, the teacher may use the Websites listed below
with a computer and LCD projector.
The nuclear power plant demonstration and a graphic of the design may be accessed
at the following URL addresses:
http://www.howstuffworks.com/nuclear-power2.htm
http://www.nrc.gov/reading-rm/basic-ref/students/reactors.html
10. Ask students to write a summary of their observations and develop questions relating
to the animation and information.
11. Share the questions with classmates and discuss to ensure understanding.
12. Ask students to use texts and other resources to develop a time line for the
advancement of nuclear energy.
Assessments:
Venn diagram, Lesson reflections/summaries, and time line
Modification(s):
The following modifications may be made to accommodate ESE and LEP students:
• Graphic organizers- Pair students prior to instruction for brainstorming about
main idea and vocabulary
• Cooperative grouping- Grouping may provide additional peer assistance with
vocabulary, as will having students write a sentence or draw a picture for each
word. Remedial strategies include journaling and writing reflections/summaries
of activities
• Visual Interpretations- Use pictures, animations, diagram and graphs.
• Spanish-English dictionaries and materials - There are also Websites where
terms may be translated
• Accommodations as listed on IEPs
Lesson Three: Worksheet One
Fission - Fusion Anticipation Guide
Instructions: Respond to each statement twice: once before the lesson and again after
reading it.
• Circle True if you agree with the statement.
• Circle False if you do not agree with the statement.
Before Reading
True False As soon as the nucleus captures the neutron, it
splits into two lighter atoms and “throws off” two
or three new neutrons.
True False Nuclear fusion is the joining (or fusing) of the
nuclei of two atoms to form a single heavier atom.
True False For fusion to occur, the electrostatic repulsion
between the atoms must be overcome.
True False The energy released by a single fission comes from
the fact that the fission products and the neutrons,
together, weigh less than the original U-235 atom.
True False The process of capturing the neutron and splitting
of an atom happens very quickly, on the order of
picoseconds (2x10-12 seconds).
True False The two atoms that result from the fission later
release alpha and gamma radiation of their own, as
well.
True False Three-percent Uranium enrichment is sufficient for
use in a civilian nuclear reactor, used for power
generation.
True False The uranium bundle acts as an extremely highenergy
source of heat. It heats the water and turns it
to steam.
True False Weapons-grade uranium is composed of 90-
percent or more U-235.
True False The probability of a U-235 atom capturing a
neutron as it passes by is fairly low.
Lesson Three: Worksheet Two
After Reading
True False As soon as the nucleus captures the neutron, it
splits into two lighter atoms and “throws off” two
or three new neutrons.
True False Nuclear fusion is the joining (or fusing) of the
nuclei of two atoms to form a single heavier atom.
True False For fusion to occur, the electrostatic repulsion
between the atoms must be overcome.
True False The energy released by a single fission comes from
the fact that the fission products and the neutrons,
together, weigh less than the original U-235 atom.
True False The process of capturing the neutron and splitting
of an atom happens very quickly, on the order of
picoseconds (2x10-12 seconds).
True False The two atoms that result from the fission later
release alpha and gamma radiation of their own, as
well.
True False Three-percent Uranium enrichment is sufficient for
use in a civilian nuclear reactor, used for power
generation.
True False The uranium bundle acts as an extremely highenergy
source of heat. It heats the water and turns it
to steam.
True False Weapons-grade uranium is composed of 90-
percent or more U-235.
True False The probability of a U-235 atom capturing a
neutron as it passes by is fairly low.
Lesson Four
Lesson Plan Four
Teacher: Angela Schneider Course: Physics Grade: 11-12
Topic: Nuclear Energy
CLE(s): Describe how changes in the nucleus of an atom during a nuclear reaction (i.e., nuclear decay, fusion, fission) result in emission of radiation).
Identify the role of nuclear energy as it serves as a source of energy for the Earth, stars, and human activity (e.g., source of electromagnetic radiation, thermal energy within mantle, nuclear power plants, fuel for stars).
Distinguish between renewable and nonrenewable energy resources
Identify and describe major scientific and technological challenges to society and their ramifications for public policy (e.g., global warming, limitations to fossil fuels, genetic engineering of plants, space and/or medical research)
Objectives: The student will identify the two different types of nuclear power plants discussed.
The student will discuss the process of energy generation with nuclear power plants.
Materials needed: overhead projector, Franklin’s Core transparency, reactor fuel assembly transparency, turbine transparency, two worksheets for each student, post-discussion questions
Introduction:
We have been studying radioactivity, as well as, nuclear fusion and nuclear fission. Today we are going to focus on the nuclear power plant, where nuclear energy is produced.
Content:
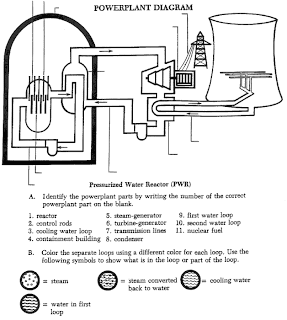
Nuclear reactor has four main parts: the uranium fuel assemblies, the control rods, the coolant/moderator, and the pressure valve. The fuel assemblies, control rods, and the coolant/moderator make up the reactor core, which is surrounded by the pressure valve, known as Franklin’s Core. Show Franklin’s Core transparency.
To produce the uranium fuel assemblies pellets of uranium are inserted into rods. These rods are then carefully bound into fuel assemblies, each of which contains around 240 rods. The assemblies hold the rods in place so that water can flow between them. Show reactor fuel assembly transparency.
The uranium undergoes a nuclear chain reaction, producing heat, which is transferred from the reactor core to the steam generator where it turns the turbine, producing electricity. Show turbine transparency.
As you know, the production of nuclear energy also produces large amounts of radiation. Therefore, it is necessary to have a plan in case there is an emergency.
The control rods are present to regulate the amount of nuclear energy produced by absorbing neutrons. Thus, fewer neutrons hit the uranium atoms, slowing down the nuclear chain reaction. They are only used when necessary.
There are two types of nuclear power plants common in the United States.
The boiling water reactor generates electricity the same way that fossil fuels do, without a steam generator. Water inside the boiling water reactor boils inside a pressure valve where a steam-water mixture is produced. When pure water (reactor coolant) moves upward through the core it absorbs heat causing the water to boil and produces steam. When the steam rises to the top of the pressure vessel, water droplets are removed, the steam is sent to the turbine generator to turn the turbine.
The pressurized water reactor differs from the BWR in that the steam to run the turbine is produced in a steam generator. Water boils at 212°F or 100°C. As the pressure increases, so does the temperature of the water in the pot. In the PWR plant, a pressurizer unit keeps the water that is flowing through the reactor vessel under very high pressure to prevent it from boiling. The hot water then flows into the steam generator where it is converted to steam. The steam passes through the turbine which produces electricity. About two-thirds of the reactor power plants in the U.S. are of the PWR type.
Conclusion:
Your assignment today is to answer the post-discussion questions (these will probably require some internet research) and complete the two nuclear power plant worksheets.
Lesson Four: Post Discussion Questions
Post-Discussion Questions
1.Is there a nuclear power plant near where you live? What type is it?
2.Why don't boiling water reactors have steam generators?
3.What is the purpose of a "cooling tower"?
4.What percentage of the electricity in the U.S. is produced in nuclear power plants?
5.Name the two types of reactor power plants in operation the U.S. What are the basic differences?
Lesson Five
Lesson Plan Five
Teacher: Angela Schneider Subject: Physics Grade: 11-12
Topic: Nuclear Energy
GLE: Identify the role of nuclear energy as it serves as a source of energy for the Earth, stars, and human activity (e.g., source of electromagnetic radiation, thermal energy within mantle, nuclear power plants, fuel for stars)
Identify and evaluate advantages/disadvantages of using various sources of energy (e.g., wind, solar, geothermal, hydroelectric, biomass, fossil fuel) for human activity
Describe how changes in the nucleus of an atom during a nuclear reaction (i.e., nuclear decay, fusion, fission) result in emission of radiation)
Distinguish between renewable and nonrenewable energy resources
Identify and describe major scientific and technological challenges to society and their ramifications for public policy (e.g., global warming, limitations to fossil fuels, genetic engineering of plants, space and/or medical research)
Objectives: The student will contribute to the class discussion at least twice
Materials needed: Class list
Phase 1: Clarify aims and establish set
We have been discussing nuclear energy and how it is produced.
Phase 2: Focus the discussion
Today we are going to discuss the pros and cons of the usage of nuclear energy. This is a controversial issue in U.S. politics, due to the dangers associated with nuclear energy. Be sure to consider environmental issues along with those associated with politics and safety.
Phase 3: Hold the discussion
As students contribute the teacher or designated student will make note of it on the class list.
Possible Questions:
(Knowledge) How is nuclear energy produced?
(Comprehension) How does the production of nuclear energy compare with some of the other energy forms currently used?
(Application) Would an increased reliance on nuclear energy be possible in the United States?
(Analysis) How would the increased usage of nuclear energy benefit the United States?
(Synthesis) If nuclear more nuclear energy usage is implemented, what will the government do with the resulting waste?
(Evaluation) Do you feel that nuclear energy is a adequate alternative source of energy?
Phase 4: End the discussion
Today we have considered some of the positive and negative arguments behind the usage of nuclear energy. This is and will continue to be an important issue.
Phase 5: Debrief the discussion
For homework I would like you to write an essay that is either for or against nuclear power. This will be in place of a test grade.
Subscribe to:
Comments (Atom)